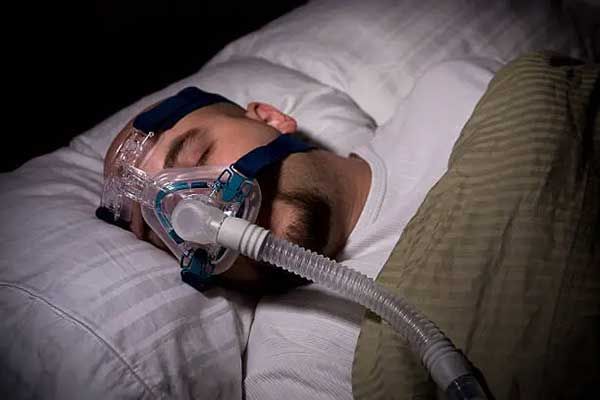
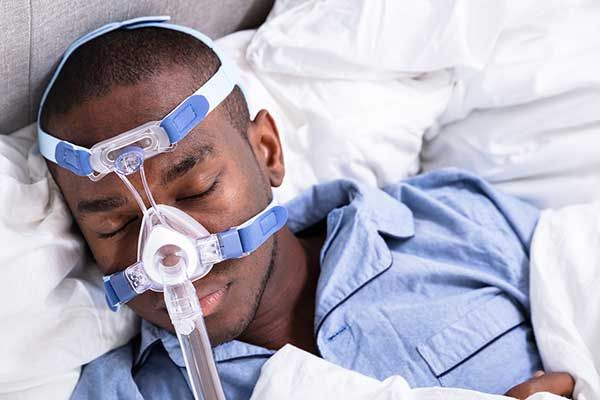
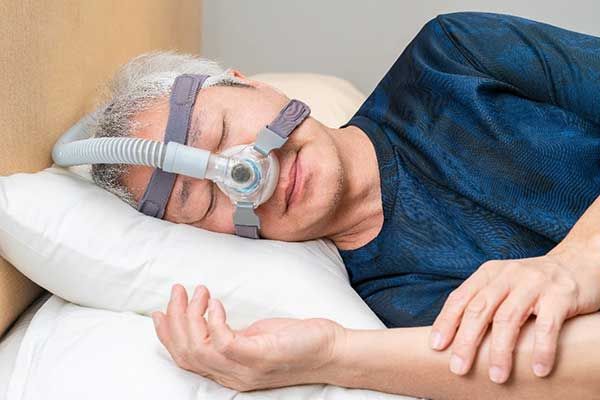
Philips has currently halted U.S. sales of its defective ventilators and CPAP machines since they recently agreed to a consent decree with the FDA...
Bard PowerPort Bellwether Trials Press On, Despite Bard’s Request for Delay
Roughly 115 lawsuits have been filed so far in the Bard PowerPort MDL alleging serious infections, blood clots, and other injuries. Bard has attempted...
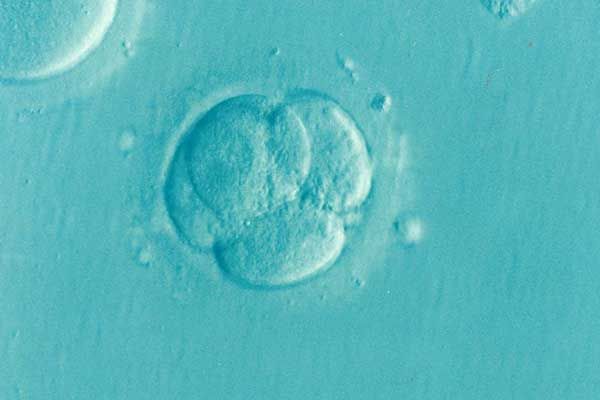
Hopeful Couples’ Dreams of Having a Family Dashed by CooperSurgical’s Defective Growth Media
Couples across the country are working through their grief after losing IVF embryos grown in defective CooperSurgical embryo culture media. Lawsuits...
CooperSurgical’s Defective Embryo Culture Solution Killed Viable Embryos, Lawsuits Claim
Plaintiffs allege IVF solution made by fertility and women's health company CooperSurgical was defective, and caused embryo death. Devastated parents...
Camp Lejeune Vets Stationed Between 1975 and 1985 at Increased Risk for Eight Cancers, Study Finds
Federal study finds that those living at Camp Lejeune between 1975 and 1985 had higher risk for eight different cancers due to contaminated water, which...
FDA Has Received 561 Reports of Death Linked to Recalled Philips PAP Devices
Since 2021, the FDA has received over 500 reports of death linked to recalled Philips CPAP machines, BiPAP machines, and certain ventilators. Other...
HIV Drugmaker Gilead to Face Negligence Claims Concerning Bony and Kidney Injuries
Gilead must face 24,000 claims in TDF HIV medication proceedings, according to appellate court ruling rejecting the company’s pleas for immunity.
Lawsuits Claim Risk of Severe Complications from Bard PowerPorts Were Hidden From Consumers
Patients have filed lawsuits against Becton Dickinson and Company and its C.R. Bard and Bard Access Systems subsidiaries alleging that the companies...
Philips OK’d Continued Sales of CPAP Machines Despite Knowledge of Defect, According to Investigation
Philips continued to sell defective breathing machines, even after its own scientists raised red flags. A year-long investigation by ProPublica and...