
Grant & Eisenhofer P.A. | Complex & Mass Tort Litigation
Current Litigation
Depo-Provera Lawsuit
Women across the country are suing manufacturers of Depo-Provera (medroxyprogesterone acetate) upon receiving devastating diagnoses of brain or spinal tumors known as meningiomas. These women had been injected with Depo-Provera, a hormonal birth control taken as a shot every three months. Plaintiffs claim the drug makers were aware of Depo-Provera’s risks, but they were never informed.
CooperSurgical Embryo Solution Lawsuit
Lawsuits have been filed against fertility company CooperSurgical claiming that a liquid used in the in vitro fertilization (IVF) process destroyed developing embryos. Plaintiffs claim that the company failed to conduct proper testing of the solution, known as embryo culture media, and that the defective product along with CooperSurgical’s negligence are to blame for their losses.

Bard PowerPort Lawsuit
Bard PowerPort lawsuits have been filed by patients across the country who claim they sustained injuries from their totally implantable vascular access device (TIVAD). Defective design of the implantable port catheter has led to fracturing, migration, or rotation after insertion for some patients, which can lead to serious infections, blood clots, other health consequences, or emergency surgery to remove the device.
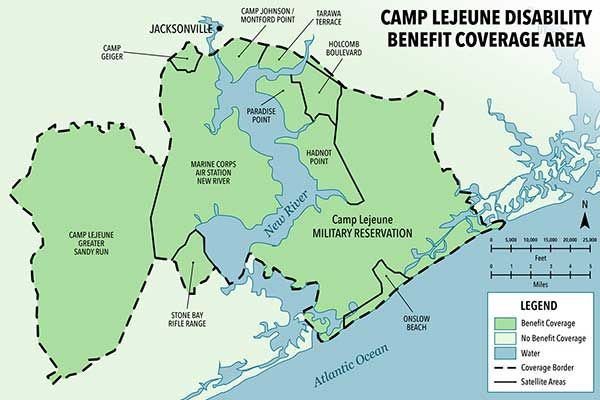
Camp Lejeune Water Contamination Lawsuit
For 35 years—between 1953 and 1987—nearly one million military service members, civilian workers, and their families lived on Marine Corps base Camp Lejeune in North Carolina, unknowingly drinking and bathing in water that was contaminated with toxins. Oil, petrol, industrial wastewater, and other chemicals were knowingly dumped into local storm drains by the U.S. government. The water contamination at Camp Lejeune has been linked to many different serious, sometimes terminal, injuries.

Tenofovir (TDF) HIV Drug Lawsuit
TDF drugs are linked to severe side-effects such as chronic kidney disease, bone density loss and fracturing. Biotechnology company Gilead Sciences, Inc. is being sued over accusations that it withheld a safer alternative to TDF to reap profits from booming sales.

Paraquat Lawsuit
Paraquat is a toxic chemical herbicide that has been used worldwide to control weed and grass growth. Exposure to paraquat has been linked to Parkinson’s disease. Lawsuits have been filed on behalf of farmers and other workers who were exposed to paraquat and subsequently developed the central nervous system disorder. Plaintiffs claim that they were not warned of the alleged dangerous health risks associated with the herbicide.

Baby Powder (Talc) Lawsuit
Studies have shown that the use of talcum powder may increase a woman’s risk of developing ovarian cancer. Often termed the “silent killer,” ovarian cancer is considered one of the most deadly cancers in women. Cases have been filed nationwide against talcum powder manufacturers as well as talc mining companies who have are alleged to have failed to warn female consumers of the risks of genital dusting.
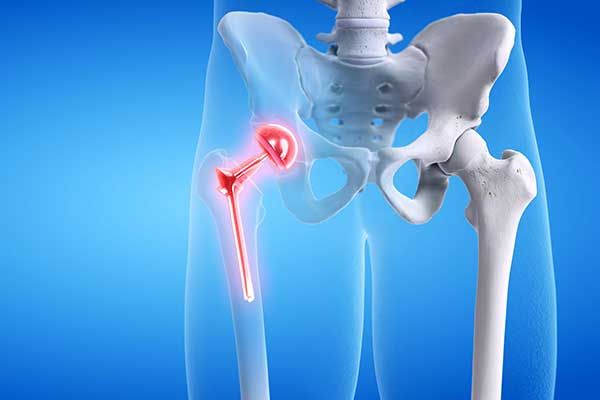
Hip Implant Lawsuit
It is estimated that over 500,000 people received metal-on-metal hip implants over the last 10 years in the U.S. alone. The dangers associated with these devices—which fail at an alarming rate—are well documented. The metal-on-metal movement inside the human body can cause the release of tiny metal particles, damaging the surrounding soft tissue and bone, and sometimes entering the bloodstream causing other systemic health issues. Failure often requires revision surgery to remove or replace the implant.
Discuss Your Potential Claim With Our Attorneys Today
If you have suffered an injury due to a defective drug, medical device, or an environmental catastrophe, please contact us at (888) 984-7988 to discuss your potential case with a G&E attorney.
