IVF Lawsuit Filed in North Carolina Over CooperSurgical’s Defective Growth Solution
A couple from North Carolina has sued CooperSurgical, seeking compensation for damages after their 13 embryos fertilized via IVF were placed in the company’s recalled embryo culture media and were damaged.
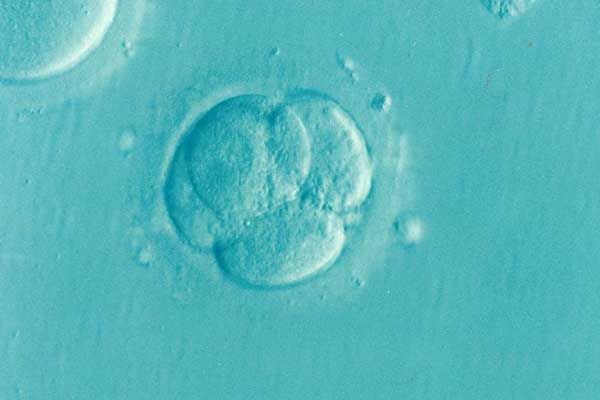
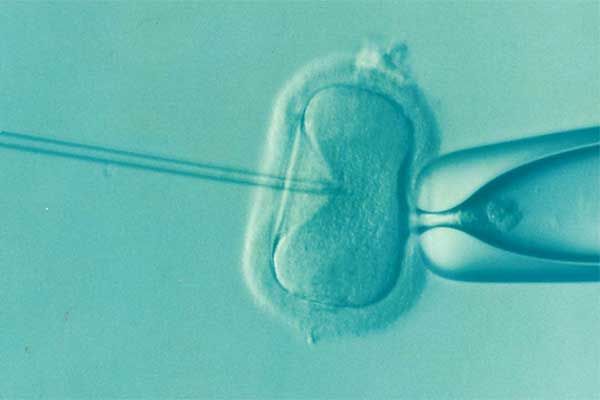
After years of fertility struggles, a North Carolina couple turned to in-vitro fertilization with hopes of starting a family. IVF is an assistive reproductive technology that has been used for decades to help couples conceive a child. A woman’s egg is retrieved, and fertilized with a man’s sperm to make an embryo. Once grown to what is known as the blastocyst stage, the embryo is implanted back into the women’s uterus to be carried to term. The process must be overseen with the utmost safety and care, as the process is time consuming, costly, and emotionally taxing—and the resulting embryos are irreplaceable.
For the plaintiffs filing this recent lawsuit, the woman’s egg retrieval was completed in November 2023. 27 eggs were retrieved, and from those, 13 were fertilized with her husband’s sperm. The embryos were then placed in LifeGlobal culture media from CooperSurgical, where they were frozen after three days of exposure to the media—before they could develop into viable blastocysts. The culture media is intended to assist the embryo’s growth for several days until it has reached the blastocyst stage. However, the solution that the couple’s precious embryos were placed in was recalled just days later, due to a sudden increase in complaints about the product. CooperSurgical said that the recalled media impaired embryo development prior to the blastocyst stage.
In December 2023, CooperSurgical recalled certain lots of its embryo culture solution that had been distributed to fertility clinics in more than 30 states and about 25 countries around the world, according to the FDA’s global recall notice posted in February 2024. Nearly 1,000 bottles of the culture media were affected, and almost half of those bottles had been distributed within the United States. Potentially thousands of patients may have been impacted by the defective solution. For many couples with dreams of becoming parents, their journey has been cut short due to alleged negligence on part of CooperSurgical.
Plaintiffs across the country have filed lawsuits against CooperSurgical, alleging, among other claims, that it failed to properly monitor their manufacturing systems and processes, which led to the culture media being manufactured without critical nutrients that embryos need to grow. Plaintiffs also contend that the company failed to adequately test or inspect the recalled batches of growth solution until after complaints were received from fertility clinics that the LifeGlobal media was rapidly destroying embryos.
These couples are devastated to lose their embryos after undergoing months of preparations, enduring emotional stress and mental fatigue, and footing expensive treatments to undergo IVF—only to have their most sacred possessions be destroyed by a defective product.
Did Your Embryos Fail to Develop in CooperSurgical’s Recalled Culture Media?
If you or a loved one lost an embryo cultivated in CooperSurgical’s recalled defective embryo solution, you may have a claim. Grant & Eisenhofer’s mass tort attorneys Beth Graham, Sindhu Daniel, and Samantha Mertz are experienced in litigation involving women’s health—and specifically reproductive issues—having represented thousands of women in litigation the Essure contraceptive device, power morcellators, and talcum powder. Contact us today to learn about your rights and what potential recoveries may be available to you to pursue due to this recall. We are a team of dedicated attorneys here for you during this difficult time.